mf6rtm: Reactive Transport Model with MODFLOW 6 and PHREEQCRM
Integration Overview
mf6rtm couples the modular capabilities of MODFLOW 6 (MF6) with the geochemical modeling power of PHREEQCRM (PHREEQC-3). The workflow is as follows:
- Initialization: PHREEQCRM and arrays are initialized using a
phinp.datfile. - Model Setup: Grid dimensions and transported components are derived from the MF6
disordisvobject and PHREEQCRM respectively. MF6 model files are written for each component. - Transport Loop: At each time step, MF6 solves the transport system. Convergence iterations and failures are tracked.
- Reaction Calculations: If enabled, concentrations are passed to PHREEQCRM for reaction calculations, and results are fed back into MF6.
- Output: MF6 handles UCN outputs. Custom outputs can also be saved as CSV.
Current Capabilities
- Structured grids (
disobject) and unstructured grids through discretization by vertices (disv) - Single simulation setup (flow + transport)
- Supported PHREEQC reactions:
- Solution
- Equilibrium phases
- Cation exchange
- Surface complexation
- Kinetics
Benchmarks
Example 1: Engesgaard & Kipp (1992)
A 1D domain with calcite and dolomite equilibrium reacting to a chemical change in inflow. Simulated using the WEL and SSM packages. Compared against PHT3D.
Example 2: Walter et al. (1994) — 1D Redox Problem
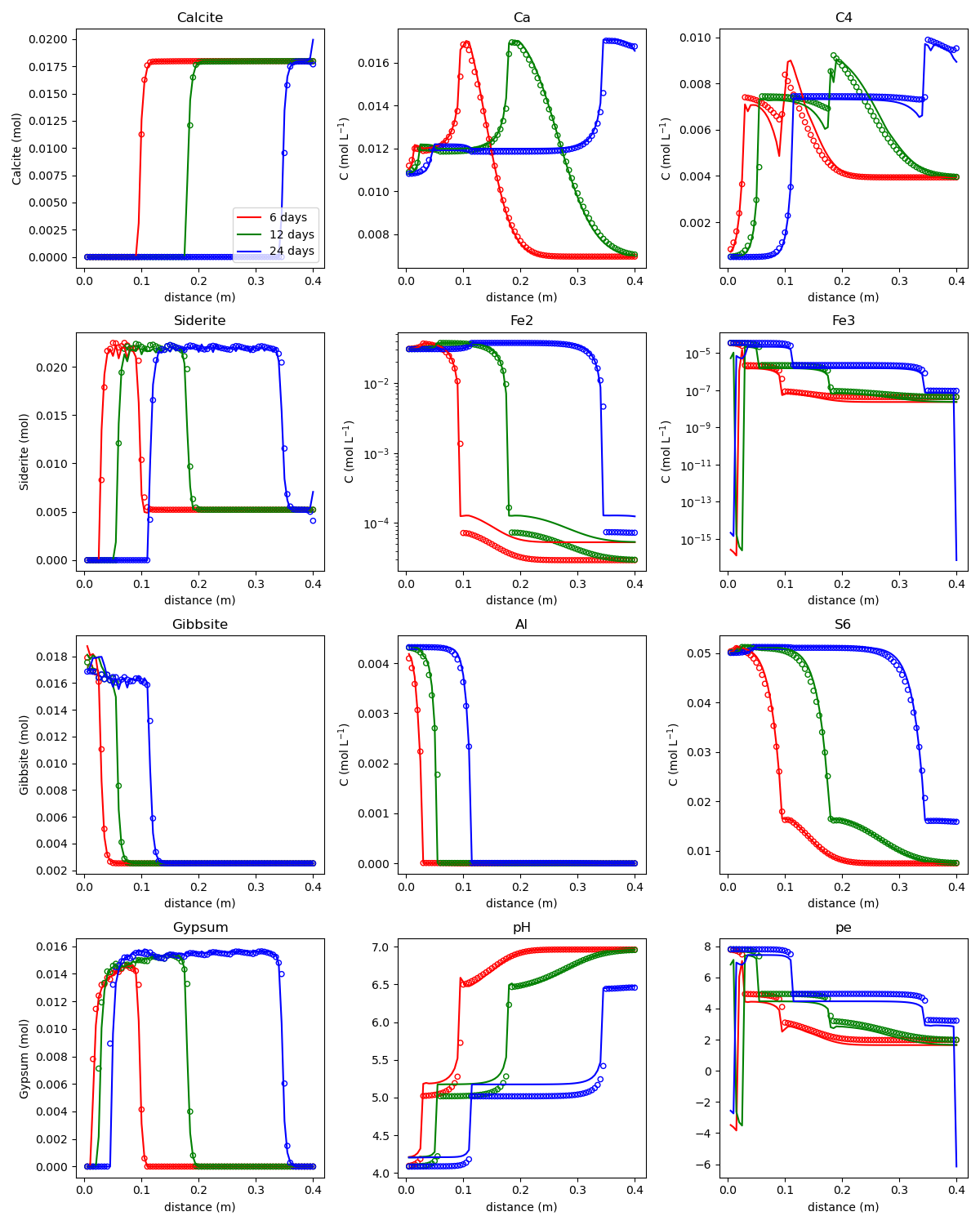
Simulates acidic mine tailings leaching into a carbonate aquifer. Includes 17 aqueous components, 6 minerals. mf6rtm uses larger time steps than PHT3D for similar accuracy.
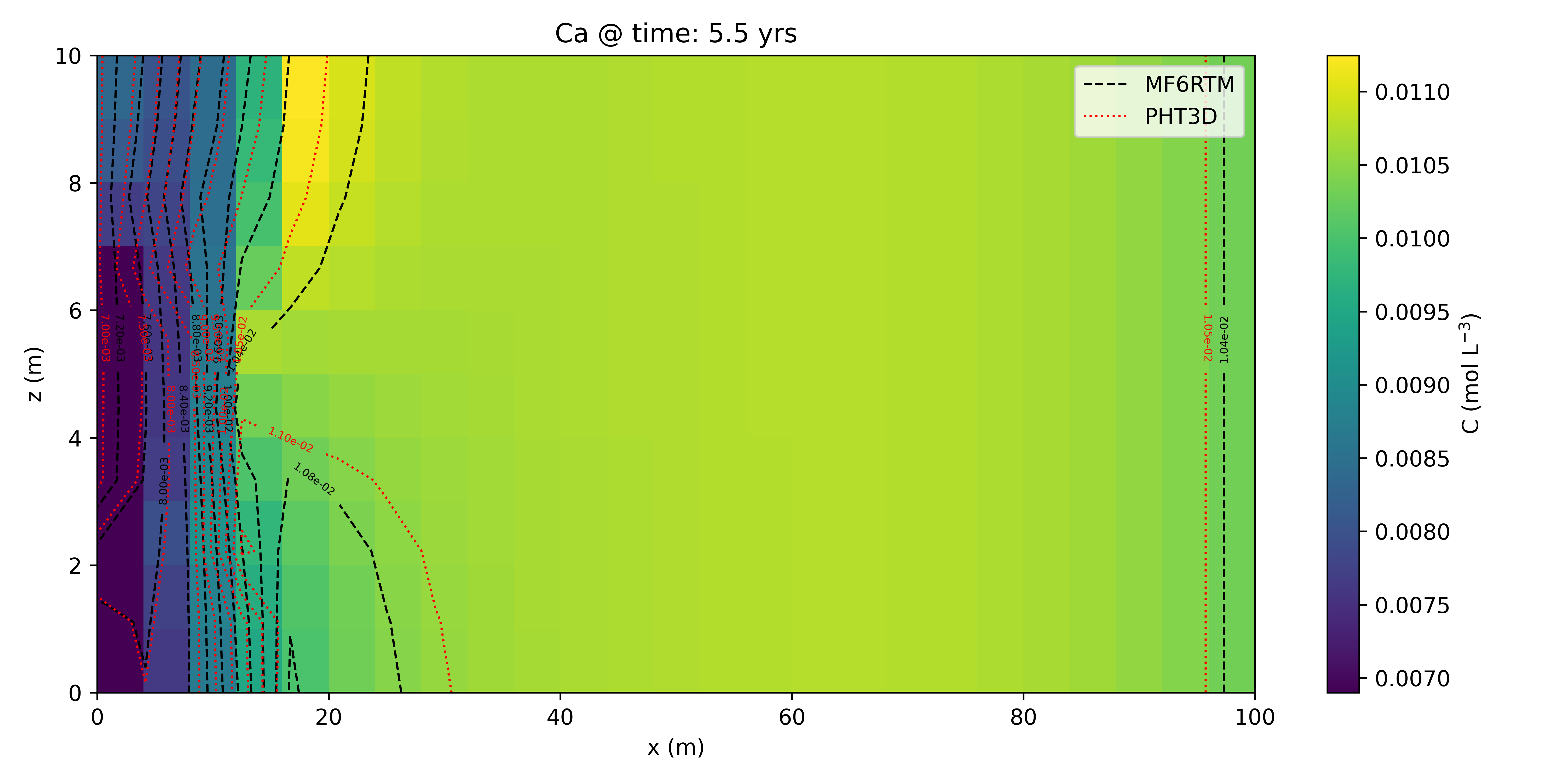
Example 3: Walter et al. (1994) — 2D Redox Problem
Extension of Example 2 to 2D. Uses CHD and CNC in MF6 for compatibility with the original PHT3D setup.

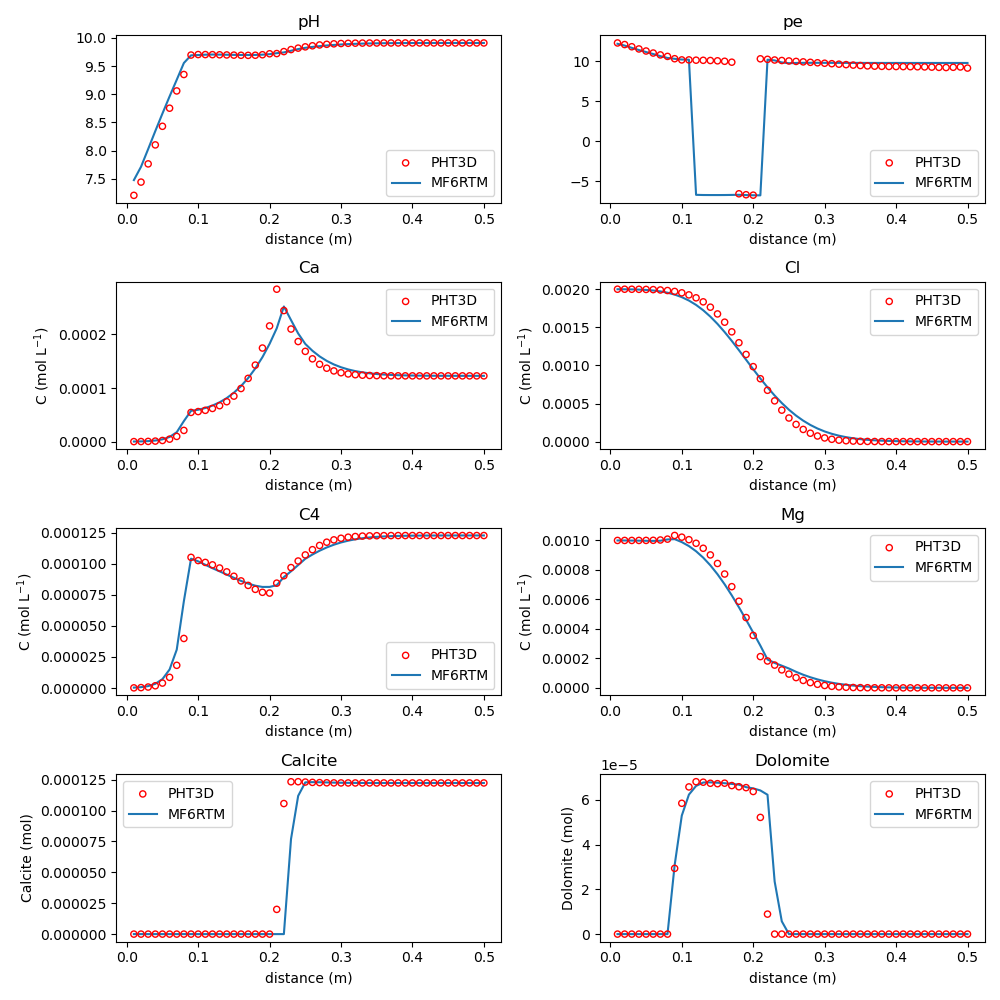
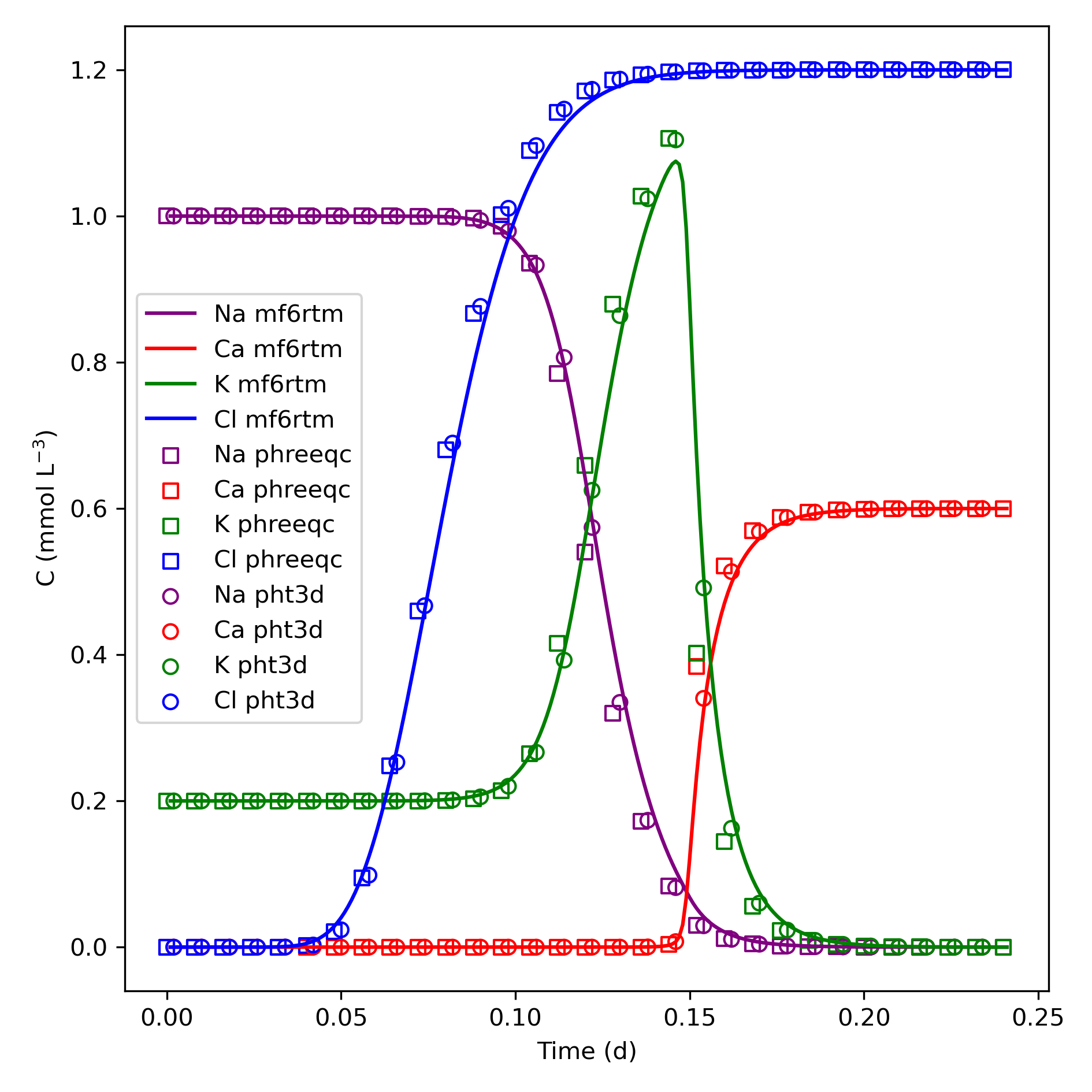
Example 4: Cation Exchange — Na/K/NO₃ flushed with CaCl₂
Simulates ion exchange in a flow-through column. Benchmarked against PHT3D and PHREEQC-3. mf6rtm uses double the time step due to numerical dispersion.
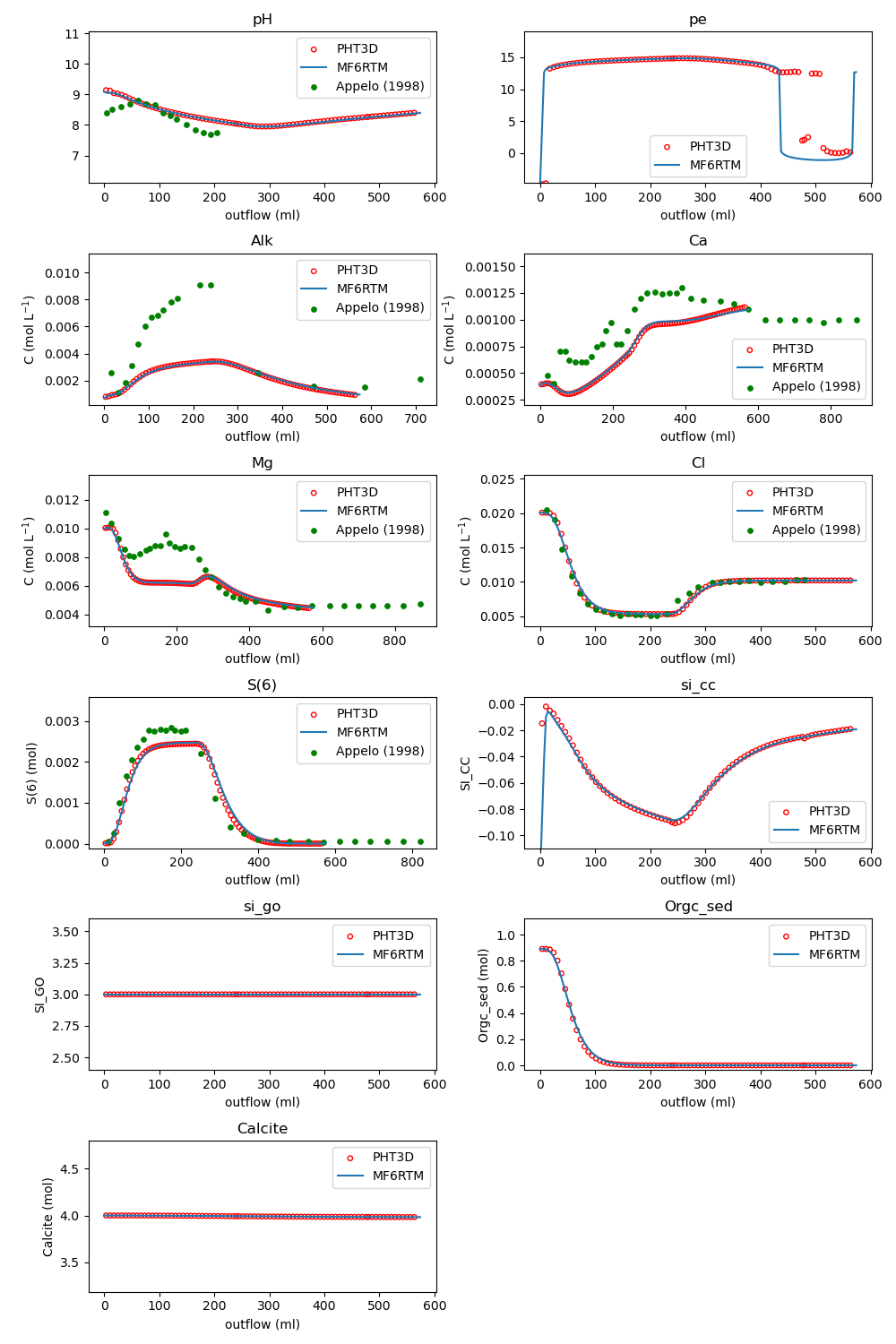
Example 5: Oxidation of Marine Pyritic Sediment (Appelo et al. 1998)
Includes pyrite oxidation, calcite dissolution, CO₂ sorption, ion exchange, and organic matter oxidation. Simulates three experimental phases involving MgCl₂ and H₂O₂ solutions.